Location: Home >> Detail
TOTAL VIEWS
Crop Breed Genet Genom. 2025;7(3):e250011. https://doi.org/10.20900/cbgg20250011
1 Horticulture & Natural Resources, Kansas State University, Manhattan, KS 66506, USA
2 Department of Plant Breeding and Genetics, University of Agriculture Faisalabad, Ghulam Muhammad Abad 38000, Pakistan
* Correspondence: Atiqa Rehman
Genetic transformation plays a vital role in enhancing crop performance under changing climatic conditions. However, soybean (Glycine max L.) remains a transformation-recalcitrant species, with regeneration efficiency strongly influenced by genotype, explant type, and in vitro culture conditions. In this study, four soybean genotypes: Faisal Soybean, AARI Soybean, Rawal-I, and Williams-82 were evaluated for their regeneration potential via direct organogenesis using half-split cotyledonary explants. Explants were cultured on Murashige and Skoog (MS) medium supplemented with varying concentrations of benzyl amino purine (BAP) and naphthalene acetic acid (NAA) for shoot induction, and indole-3-butyric acid (IBA) and indole-3-acetic acid (IAA) for root induction. Among the tested genotypes, Faisal Soybean exhibited the highest regeneration efficiency, with 40.13% shoot induction and 26.83% root induction, followed by AARI Soybean. The optimal shoot induction was achieved on MS medium containing 2.0 mg·L−1 BAP + 0.1 mg·L−1 NAA, while the highest root induction was observed on medium with 0.5 mg·L−1 IBA + 1.2 mg·L−1 IAA. In contrast, Rawal-I showed the lowest regeneration response across all stages. These findings highlight significant genotype-dependent variation in regeneration potential, reinforcing the importance of selecting responsive cultivars for transformation. The optimized protocol developed in this study provides a reliable foundation for future genetic transformation and crop improvement efforts in soybean.
Soybean (Glycine max L.), originally domesticated in China, has become a globally important legume crop, cultivated extensively across North and South America as well as Asia [1]. Its significance stems not only from its vast cultivation area and high production values but also from its role as a staple food crop and a primary source of protein and oil [2]. Soybeans are utilized in both fermented and non-fermented food products, such as tofu, soymilk, soy yoghurts, and soy cheese, offering considerable nutritional and health benefits [3]. The crop’s production has increased fivefold over the past forty years, a trend expected to continue due to rising demand for food, feed, and biofuel.
Despite this growth, soybean improvement faces considerable challenges. Traditional breeding methods are limited by the crop’s self-pollinating nature [4], narrow genetic base, and susceptibility to a range of biotic and abiotic stresses, particularly drought, which severely impacts yield and seed quality [5]. These constraints have driven the need for genetic transformation to introduce desirable traits such as stress tolerance, disease resistance, and enhanced nutritional value.
However, the genetic transformation of soybean is hindered by several prevailing challenges. Soybean is considered recalcitrant to genetic transformation, with low rates of transgene integration and plant regeneration, especially when compared to other crops. This is due to genotype dependency, limited explant responsiveness, and difficulties in tissue culture [6–8]. Most successful transformation protocols have been developed for a narrow set of model cultivars, with limited applicability to elite or locally adapted varieties. This restricts the broader adoption of transgenic technologies [6,8]. The influence of key factors such as explant type, age, growth regulator concentrations, and environmental conditions on regeneration efficiency remains incompletely understood, particularly for cultivars grown in diverse agro-ecological regions [6,9,10]. Many countries, including Pakistan, face unique challenges in adapting soybean to local climates. The lack of high-yielding, disease-resistant, and climate-resilient cultivars impedes the crop’s expansion and productivity [11,12].
The prerequisite for successfully deploying genetic transformation technology is an effective and reproducible protocol for plant regeneration. Despite their low transformation efficiency, Agrobacterium-based transformation systems have been used to create transgenic soybean lines resistant to pathogens such as Septoria glycines and soybean mosaic virus. Research efforts continue to enhance regeneration potential via organogenesis and somatic embryogenesis. Organogenesis, in particular, offers promise due to the availability and accessibility of explants such as cotyledonary nodes, hypocotyls, and primary leaf nodes, which have been shown to support regeneration in multiple studies [13–20].
Numerous factors such as genotype, explant source, explant age and size, plant growth regulator concentrations, basal medium composition, and environmental conditions significantly influence regeneration efficiency [13,21–24]. Yet, regeneration studies have so far concentrated on a few cultivars, while ignoring superior or widely cultivated local genotypes. In Pakistan, where soybean remains in an adaptation phase, developing regeneration protocols tailored to local cultivars is especially important. Past efforts at soybean introduction have suffered from inconsistent yields and limited success, mainly due to the lack of locally adapted, high-performing genotypes [25–27].
Although advances have been made using Agrobacterium-mediated transformation and organogenesis-based regeneration, these methods still exhibit low efficiency and are not widely applicable across different genotypes [6,9,28–30]. The majority of research has focused on a limited number of cultivars, leaving a significant gap in protocols tailored to local or superior varieties [6,8,10].
This study is specifically designed to address these knowledge gaps and practical challenges. By systematically evaluating the regeneration potential of local soybean genotypes through direct organogenesis, and optimizing key factors such as cultivar type and plant growth regulator concentrations, this research aims to expand the understanding of genotype-specific responses in tissue culture and regeneration, develop a robust, reproducible protocol for direct organogenesis-based regeneration applicable to local cultivars, and provide a methodological benchmark for future genetic transformation efforts in soybean, particularly for regions with unique climatic and agronomic conditions, such as Pakistan. By targeting these critical issues, the study not only contributes to the scientific understanding of soybean regeneration but also lays the groundwork for the development of improved, climate-adapted, and high-yielding soybean cultivars through genetic transformation.
The research was conducted at the Soybean Lab, Center for Advanced Studies in Agriculture and Food Security (CAS-AFS), University of Agriculture Faisalabad.
Plant MaterialWe got seeds of the cultivars “Faisal soybean” and “AARI soybean” from the Ayub Agriculture Research Institute (AARI), Faisalabad, to maximize organogenesis in soybean. The variety “Rawal-I” was collected from National Agricultural Research Center (NARC), Islamabad whereas “Williums-82” was obtained from US Department of Agriculture and was used as part of this research. Seeds of these varieties were stored in airtight containers at −20 °C in the Germplasm Storage Room, Soybean Lab, CAS-AFS, University of Agriculture Faisalabad.
For each genotype, 90 seeds were used to prepare cotyledonary explants. The experiment was arranged in a completely randomized design (CRD), with three independent replicates for each treatment per genotype. Each replicate consisted of 60 explants per genotype, resulting in a total of 15 explants per genotype per treatment.
Seeds Surface SterilizationSeeds were surface sterilized before using them for tissue culture. We first cleaned the seeds with tap and distilled water. Next, we treated the seeds with 50% sodium hypochlorite solution (NaClO) for 45 mins through gentle agitation by hand. Treated seeds were washed with distilled water at least three times to remove any residues of sodium hypochlorite solution. The seeds were treated with 0.1% mercuric chloride (HgCl2) for 2–3 mins. The seeds were washed three times with distilled water to remove HgCl2 residues (Figure 1A).
 Figure 1. Tissue culture process for the development of directly regenerated soybean plants (Faisal soybean as a representative); (A) surface sterilization of seeds with 50% Sodium Hypochlorite solution; (B) soaking seeds for 24–48 h in dark conditions; (C) inoculation of half-split cotyledons on shoot induction media (SIM); (D) greening of half-split cotyledons placed on SIM; (E) emergence of shoots from half-split cotyledons; (F) induction of roots of plantlets placed in rooting media; (G) hardening and acclimatization of regenerated plantlets; (H) regenerated soybean plantlets.
Figure 1. Tissue culture process for the development of directly regenerated soybean plants (Faisal soybean as a representative); (A) surface sterilization of seeds with 50% Sodium Hypochlorite solution; (B) soaking seeds for 24–48 h in dark conditions; (C) inoculation of half-split cotyledons on shoot induction media (SIM); (D) greening of half-split cotyledons placed on SIM; (E) emergence of shoots from half-split cotyledons; (F) induction of roots of plantlets placed in rooting media; (G) hardening and acclimatization of regenerated plantlets; (H) regenerated soybean plantlets.
The seeds were soaked in distilled water for 24–48 h at 23 °C in the dark (Figure 1B). The imbibed seeds were then longitudinally cut along the hilum using an autoclaved scalpel after removing the seed coat, producing the half-split seed cotyledonary explants (under sterile conditions).
Explant InoculationThese cotyledonary explants were then positioned on solidified shoot induction media (SIM: MS salts with Vitamins 4.43 g/L, sucrose 30 g/L, Phytagel 8 g/L, BAP 1 mg/L (Treatment-1), BAP 2 mg/L (Treatment-2), BAP 3 mg/L (Treatment-3) and BAP 2 mg/L + NAA 0.1 mg/L (Treatment-4) maintained at pH 5.6) in petri dishes using autoclaved forceps under sterile conditions (Figure 1C). The petri dishes were then placed in a growth chamber after sealing them with parafilm at a temperature range of 26–30 °C for two weeks (Figure 1D). The induced shoots from the cotyledonary explants were transferred to shoot elongation media (SEM: MS salts with Vitamins 4.43 g/L, sucrose 30 g/L, Phytagel 8 g/L, BAP 0.5 mg/L and gibberellic acid 0.6 mg/L) for further proliferation of shoots for two weeks under same conditions (Figure 1E). The healthy shoots were transferred to rooting media (RM: MS salts with Vitamins 4.43 g/L, sucrose 30 g/L, Phytagel 8 g/L, IBA 2 mg/L + IAA 0.5 mg/L (Treatment-5), IBA 1.5 mg/L + IAA 0.5 mg/L (Treatment-6), IBA 1 mg/L + IAA 1.2 mg/L (Treatment-7) and IBA 0.5 mg/L + IAA 1.2 mg/L (Treatment-8) to induce roots in culture jars (Figure 1F). The process was repeated for all four varieties of soybean, i.e., Faisal soybean, AARI soybean, Williums-82, and Rawal-I.
Statistical AnalysisData were analyzed statistically by using the software “Statistix 8.1” (Analytical Software, Tallahassee, FL, USA). The analysis of variance (ANOVA) technique was used to determine the overall significance of the data. Differences and comparisons were made by up to 3-way factorial LSD test at a 5% probability level. The results of these analyses determined the regeneration response of soybean genotypes. The genotype with the least time taken for regeneration, shoot initiation, and root initiation, along with a higher shoot and root induction percentage, was considered beneficial in in vitro soybean regeneration methods.
In this study, half-split cotyledon explants from four soybean genotypes were used to evaluate plant regeneration using direct organogenesis. Different genotypes reacted differently to the contamination control. Treating seeds for 20 mins with 50% NaClO did not eliminate contamination. The optimal time for contamination control was observed to be 45 mins of treatment with 50% NaClO, followed by 2 mins of treatment with HgCl2. ‘Faisal soybean’ expressed the lowest contamination rate on MS media, suggesting that it is less prone to contamination than the other genotypes under consideration (Figure 2A). ‘Faisal soybean’ also showed the highest rate of explant survival (70.5%) on MS media, followed by ‘AARI soybean,’ ‘Rawal-I,’ and ‘Williums-82’ (Figure 2B).
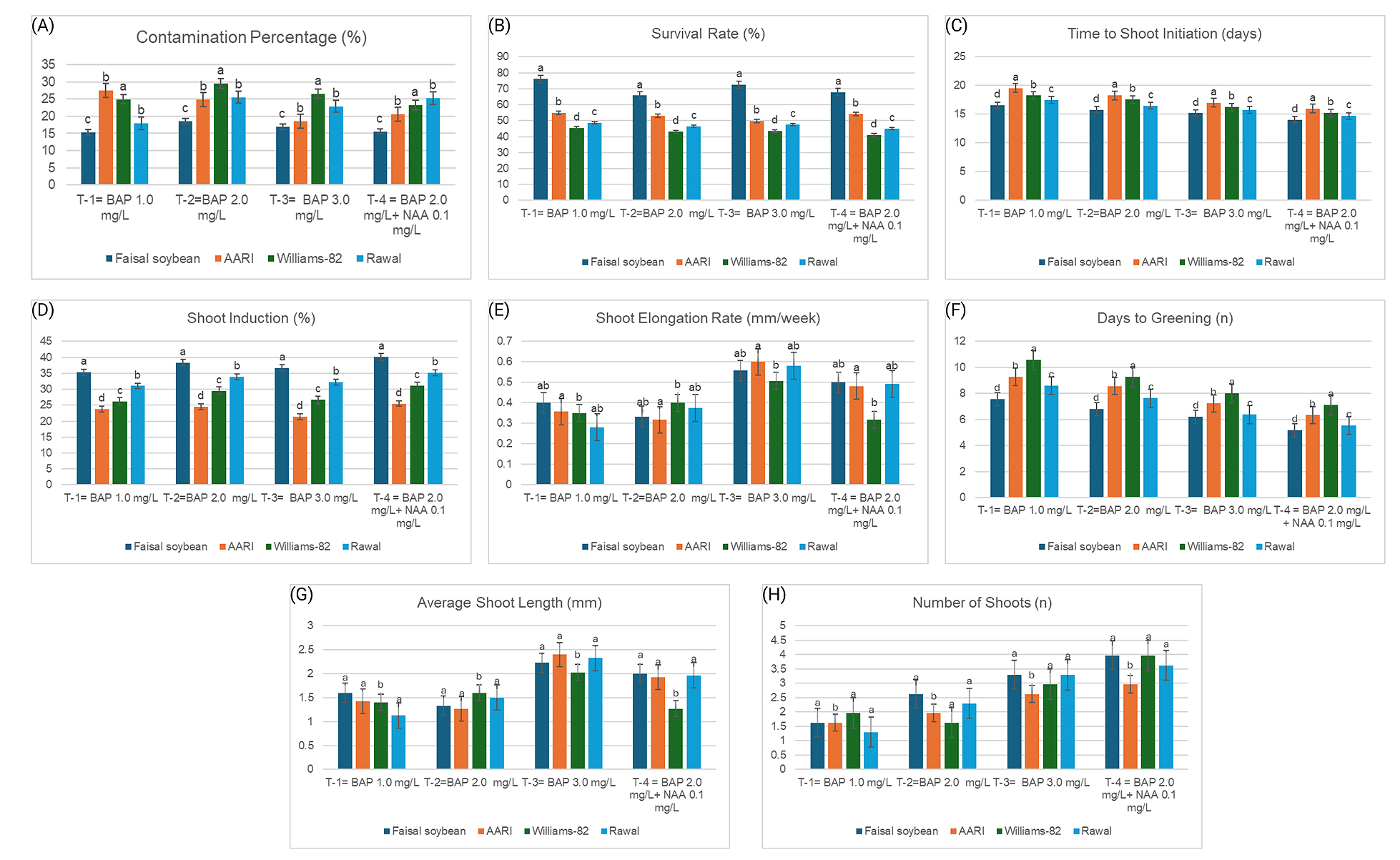 Figure 2. Response of soybean genotypes towards (A) contamination, (B) survival, (C) time to shoot initiation, (D) shoot induction, (E) shoot elongation, (F) days to greening, (G) average shoot length, and (H) the average number of shoots at different PGR treatments. On X-axis, treatments show concentrations of BAP and NAA in shooting media. Y-axis represents the average values of different parameters. Bars indicate the mean values, and the error bars represent the value of standard error (n = 3). Values were taken by applying Least Significant Difference (LSD) test (p < 0.05) at 5% level of significance. Means followed by the same lowercase letter do not differ significantly among genotypes within the same treatment composition.
Figure 2. Response of soybean genotypes towards (A) contamination, (B) survival, (C) time to shoot initiation, (D) shoot induction, (E) shoot elongation, (F) days to greening, (G) average shoot length, and (H) the average number of shoots at different PGR treatments. On X-axis, treatments show concentrations of BAP and NAA in shooting media. Y-axis represents the average values of different parameters. Bars indicate the mean values, and the error bars represent the value of standard error (n = 3). Values were taken by applying Least Significant Difference (LSD) test (p < 0.05) at 5% level of significance. Means followed by the same lowercase letter do not differ significantly among genotypes within the same treatment composition.
The time taken by a genotype for regeneration is a crucial element in determining its use for tissue culture. Ideally, a genotype should take less time to undergo organogenesis. It was noted that the ‘Faisal soybean’ took a relatively shorter period to complete its regeneration process from half-split cotyledons. On average, it took only 6 days to turn green while the values for remaining genotypes were higher than these (Figure 2F). A similar trend was seen for the shoot and root initiation time, where ‘Faisal soybean’ took less time than the other soybean genotypes (Figures 2C and 3D). This trend can be attributed to the genetic base of these varieties that allows one genotype to go under organogenesis faster than another.
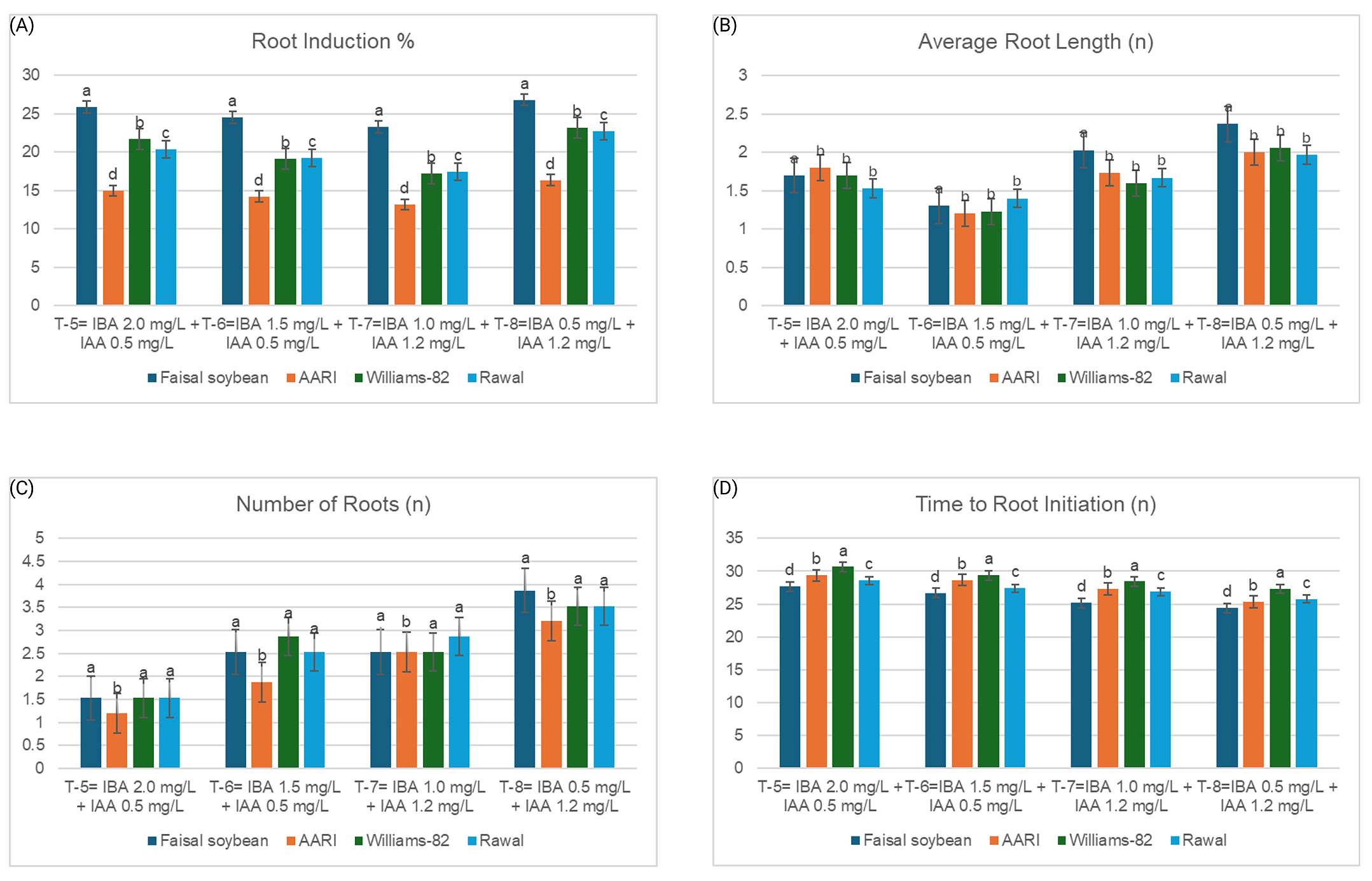 Figure 3. Response of soybean genotypes towards (A) root induction, (B) average root length, (C) the average number of roots, and (D) time to root initiation on MS rooting media at different PGR treatments. On X-axis, treatments show concentrations of IBA and IAA in rooting media. Y-axis represents the average values of different rooting parameters. Bars indicate the mean values, and the error bars represent the value of standard error (n = 3). Values were taken by applying Least Significant Difference (LSD) test (p < 0.05) at 5% level of significance. Means followed by the same lowercase letter do not differ significantly among genotypes within the same treatment composition
Figure 3. Response of soybean genotypes towards (A) root induction, (B) average root length, (C) the average number of roots, and (D) time to root initiation on MS rooting media at different PGR treatments. On X-axis, treatments show concentrations of IBA and IAA in rooting media. Y-axis represents the average values of different rooting parameters. Bars indicate the mean values, and the error bars represent the value of standard error (n = 3). Values were taken by applying Least Significant Difference (LSD) test (p < 0.05) at 5% level of significance. Means followed by the same lowercase letter do not differ significantly among genotypes within the same treatment composition
Comparing the percentages of shoot and root induction for the several genotypes studied showed a significant difference. ‘Faisal soybean’ depicted the highest percentage for shoot induction (37.6%), followed by ‘Rawal-I,’ ‘Williums-82’, and ‘AARI soybean’ at 33.0%, 28.4%, and 23.8%, respectively (Figure 2D). The trend for root induction percentage was similar. ‘Faisal soybean’ demonstrated the highest percentage for root induction, followed by ‘Williums-82’, ‘Rawal-I,’ and ‘AARI soybean’ (Figure 3A). For the number of shoots and roots regenerated per explant and the average length of shoots and roots, insignificant variation was observed between the genotypes (Figures 2G,H and 3B,C).
The potential of organogenesis tends to be influenced by the genotype of a plant. Of the four varieties used in this research, the ‘Faisal soybean’ depicted the highest potential towards organogenesis, having the highest shoot induction percentage and taking the least time for shoot initiation. Kantayos & Bae [31] have also proved this genotype-dependency of organogenesis. They investigated shoot regeneration capacity in five Korean soybean cultivars (Dawon, Daewon, Chongdoo, Pungsan, and Taekwang) through direct organogenesis of cotyledonary nodes. They found ‘Dawon’ and ‘Pungsan’ to have the most efficient shoot regeneration capacity.
The genes expressed in particular genotypes during the process of organogenesis may provide an explanation for why some varieties undergo early organogenesis than others. Li and group [32] conducted similar research, where they determined specific genes using real-time quantitative PCR and provided an insight into the somatic embryogenesis and regeneration processes. The same can be performed for the soybean varieties considered for this research using transcriptomic sequencing and real-time qPCR to understand organogenesis at a genomic and transcriptomic level.
Effect of Explant Sources on Soybean OrganogenesisThe half-seed explant is an efficient source for the soybean shoot initiation and regeneration, as was utilized in this research. The cotyledonary nodal shoots and roots generated from half-seed could be an ideal starting point for establishing an effective soybean transformation protocol. Cotyledonary nodes have been successfully utilized in the regeneration and transformation of various plants [33–36] including soybeans [37–39]. In addition to being effective for tissue culture, half-split seeds are also quicker to prepare as explants than alternative explant, such as cotyledonary nodes, whole hypocotyls, or half-split hypocotyls. Half seeds had already been used as explants in several research projects, including those by Radhakrishnan & Kumari [40] and Verma et al. [41], which discovered that they were an effective source for organogenesis.
Organogenesis of Soybeans and the Effects of Plant Growth Regulators (PGRs)The effects of the PGRs, BAP, NAA, IBA, and IAA were examined in this study in addition to the genotypes. Significant changes in the outcomes were induced by media having various concentrations of these PGRs. For instance, among all the genotypes evaluated, MS shoot induction media substituted with 2 mg/L BAP and 0.1 mg/L NAA showed the highest shoot induction percentage and produced a greater number of shoots. (Figure 2D,H). Additionally, the average shoot length was the highest in this media composition (Figure 2G). Explants growing on MS medium supplemented with 3 mg/L BAP and 2 mg/L BAP along with 0.1 mg/L NAA showed higher rates for shoot elongation than those growing on MS media with 1 mg/L BAP or 2 mg/L BAP (Figure 2E).
It has been demonstrated that adding the right quantity of BAP to tissue culture medium has a good effect on explants’ general capacity for shoot induction and organogenesis. This study determined that 2 mg/L of BAP was the optimal BAP concentration in MS medium. Other studies, including the one carried out by Biabani [35] have stressed the significance of this hormone for accelerating the rate of organogenesis. The nature of the BAP hormone can explain this effect. BAP is a cytokinin that controls differentiation, prevents the growth of roots, accelerates RNA synthesis, and boosts protein and enzyme activity in addition to controlling cell division when taken in the proper dosage. BAP thereby increases the explant’s cell proliferation and induces shoot growth when applied in the proper quantities.
To help the explant develop its roots, IBA and IAA were used in this research. Rooting media supplemented with 0.5 mg/L IBA and 1.2 mg/L IAA demonstrated the highest root induction percentage and produced a significant number of roots for all the tested genotypes (Figure 3A,C). The average root length was the highest in this composition of rooting media. These findings are in coherence with Biabani [42]. IBA and IAA belong to the class of hormones called ‘Auxins’. They control the growth of adventitious roots, the enlargement of tissues, and cell division, restrict the proliferation of auxiliary and adventitious shoots, and induce embryogenesis. When used in appropriate concentrations, they can boost the cell elongation and formation of adventitious roots and hence aid the process of rooting in organogenesis.
In this study, we optimized the direct organogenesis of half-split cotyledons protocol for soybean plant regeneration. The most effective method for direct soybean regeneration was “Faisal soybean” on MS shoot induction medium supplied with 2 mg/L BAP and 0.1 mg/L NAA and on MS rooting medium supplemented with 0.5 mg/L IBA and 1.2 mg/L IAA. As a result, the approach described here could advance soybean transformation systems further using Agrobacterium-mediated T-DNA transfer.
The optimized soybean regeneration protocol can efficiently and effectively produce genetically uniform and true-to-type soybean plants. This is important because genetically uniform plants are more predictable in terms of their growth and development, as well as their yield and quality. Additionally, true-to-type plants ensure that the desired traits, such as disease resistance or improved yield, are consistently present in the crop. Therefore, soybean regeneration protocols can provide farmers with improved crop yields, reduce the risk of crop loss, and improve the quality of their soybeans. This in turn can increase agricultural output and improve farming operation efficiency.
The dataset of the study is available from the authors upon reasonable request.
AR was responsible for the overall conceptualization of the study, developed the methodology, conducted the formal analysis, and carried out the investigation. She also managed data curation, prepared the original draft of the manuscript, and handled the visualization of the results. MAR contributed to the manuscript through review and editing. ZA provided supervision throughout the research process and oversaw project administration.
The authors declare that they have no conflicts of interest.
This study was conducted without any external funding.
Not applicable.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
Rehman A, Rafique MA, Ahmed Z. Comparative evaluation of soybean genotypes for In Vitro regeneration via direct organogenesis. Crop Breed Genet Genom. 2025;7(3):e250011. https://doi.org/10.20900/cbgg20250011.

Copyright © Hapres Co., Ltd. Privacy Policy | Terms and Conditions